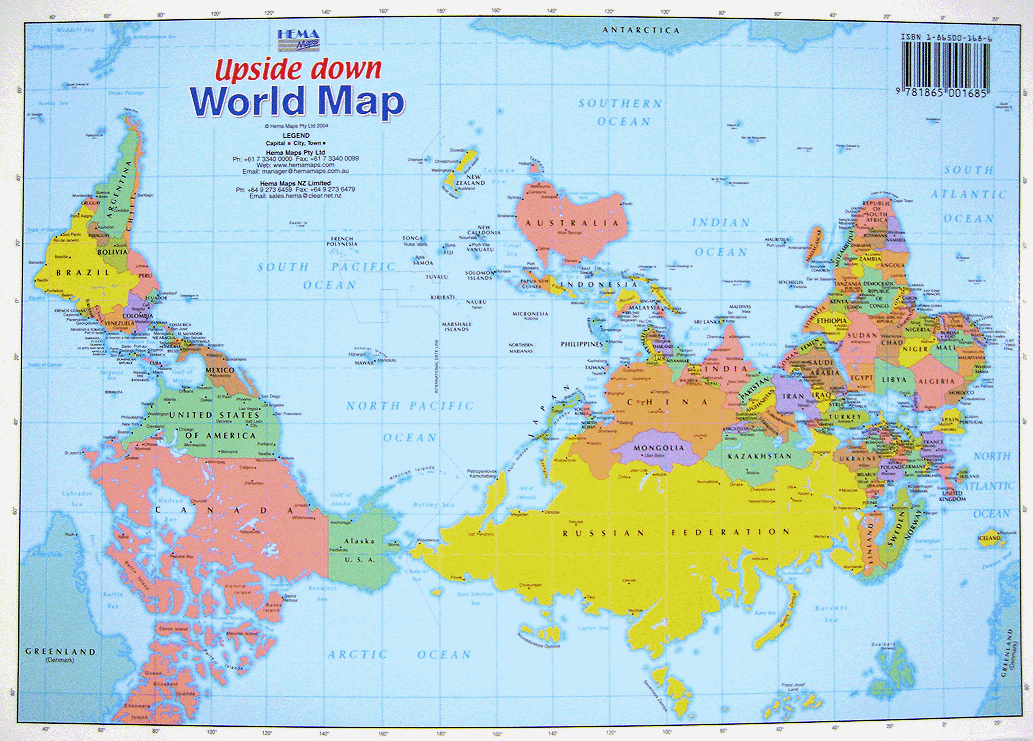
Export thread
Nerdy Musings
#26
Pojodan
Pojodan
#28
Kitty Sinatra
Kitty Sinatra
#51
Soliloquy
Soliloquy
#55
Soliloquy
Soliloquy
#60
Gill Kaiser
Gill Kaiser
#64
Gill Kaiser
Gill Kaiser
#66
Soliloquy
Soliloquy
#67
Iaculus
Iaculus
#72
Soliloquy
Soliloquy
#73
Kitty Sinatra
Kitty Sinatra
#74
Iaculus
Iaculus